Highly cross-linked polyethylene: a review of recent advances
Background / Problem First-generation highly cross-linked polyethylene (XLPE) has demonstrated excellent long-term survivorship in total hip arthroplasty (THR) by significantly reducing wear and subsequent osteolysis compared to conventional polyethylene. As this material technology continues to evolve, a comprehensive understanding of recent advancements and their clinical implications is necessary for the practicing surgeon.
Objective of the Article This review synthesizes recent and original publications on the ongoing progress of XLPE. It examines new applications in monoblock and dual mobility components, the performance of second-generation antioxidant-stabilized materials, emerging manufacturing processes, and the translation of this technology to total knee arthroplasty (TKR).
Key Points / Core Message Third-generation vitamin E-stabilized XLPE used in uncemented monoblock cups shows promising mid-term results, with low wear rates comparable to modular designs. For dual mobility constructs, second-generation XLPE (sequentially annealed or vitamin E-infused) may be biomechanically superior due to better preservation of mechanical properties. At 10-year follow-up, vitamin E-stabilized XLPE liners in THR demonstrate significantly lower radiographic wear than first-generation XLPE, although this has not yet translated to a lower revision rate. Driven by a shortage of cobalt-60, chemical cross-linking with antioxidants is emerging as a viable alternative to gamma irradiation. In contrast to THR, current evidence from meta-analyses does not support the routine superiority of XLPE over conventional polyethylene in TKR. Rare XLPE failures underscore the importance of analyzing explants and maintaining meticulous surgical technique.
Conclusion / Implications for Practice While progress in polyethylene technology is encouraging, each formulation must be evaluated individually based on long-term data. XLPE is the standard of care for primary THR, but its routine use in primary TKR is not yet supported by robust evidence. Surgeons must remain vigilant regarding implant design and positioning, as technical precision is critical to success even with advanced biomaterials.
Introduction
In 1998, the first highly cross-linked polyethylene (XLPE) implant was fitted in a total hip replacement (THR) patient with the goal of reducing wear, and subsequently osteolysis and loosening. Today, almost all THR and over 70% of total knee replacements (TKR) use one of the forms of XLPE. Cohort studies, which now have over 20 years of follow-up [1], García-Rey E, Cruz-Pardos A, Saldaña L. New polyethylenes in total hip arthroplasty: a 20- to 22-year follow-up study. Bone Joint J. 2022 Sep;104-B(9):1032-1038. [2] Orita K, Goto K, Kuroda Y, Kawai T, Okuzu Y, Matsuda S. Wear resistance of first-generation highly cross-linked annealed polyethylene in cementless total hip arthroplasty is maintained 20 years after surgery. Bone Joint J. 2022 Feb;104-B(2):200-205. , and data from national registries [3] de Steiger R, Lorimer M, Graves SE. Cross-Linked Polyethylene for Total Hip Arthroplasty Markedly Reduces Revision Surgery at 16 Years. J Bone Joint Surg Am 2018;100:1281–8. unequivocally demonstrate a marked reduction in the rates of revisions for implants using XLPE compared to those using conventional PE (CPE). Aggregations of these many results, multiple meta-analyses [4] Yoon BH, Park JW, Lee YK, Koo KH, Chang CB. Long-Term Wear-Related Complications of Cross-Linked Versus Conventional Polyethylene After Total Hip Arthroplasty: A Meta-Analysis. J Arthroplasty. 2022 Nov;37(11):2308-2315.e2. and literature reviews (also including younger patients [5] Deans CF, Buckner BC, Garvin KL. Wear, Osteolysis, and Aseptic Loosening Following Total Hip Arthroplasty in Young Patients with Highly Cross-Linked Polyethylene: A Review of Studies with a Follow-Up of over 15 Years. J Clin Med. 2023 Oct 19;12(20):6615. ) have been widely published showing excellent long-term results for first generation XLPE implants. We decided not to revisit these findings in detail, as they are now well-known. Instead, this article will review selected recent and original publications concerning this material and its ongoing progress.
XLPE in monoblock uncemented cups
The concept of the monoblock uncemented cup was first introduced in 1983 [6] Pakvis D, Biemond L, van Hellemondt G, Spruit M. A cementless elastic monoblock socket in young patients: a ten to 18-year clinical and radiological follow-up. Int Orthop. 2011;35:1445–51.. The rationale was to enable the use of a thicker polyethylene liner to prevent backside wear. Furthermore, the cup's elastic modulus, similar to that of bone, was intended to improve the load transfer to the surrounding hip socket bone, reducing stress shielding in the long term [7], Pitto RP, Bhargava A, Pandit S, Munro JT. Retroacetabular stress-shielding in THA. Clin Orthop. 2008 Feb;466(2):353-8.[8], Meneghini RM, Ford KS, McCollough CH, Hanssen AD, Lewallen DG. Bone remodeling around porous metal cementless acetabular components. [9] Brodt S, Jacob B, Nowack D, Zippelius T, Strube P, Matziolis G. An Isoelastic Monoblock Cup Retains More Acetabular and Femoral Bone Than a Modular Press-Fit Cup: A Prospective Randomized Controlled Trial. J Bone Joint Surg Am . 2021 Jun 2;103(11):992-999. . This was made from conventional polyethylene coated with titanium particles for primary bone fixation and two pegs to improve stability in rotation (Fig. 1a). These implants had an excellent survival rate of 94.4 %, with aseptic loosening used as the assessment criterion [10] Ihle M, Mai S, Pfluger D, Siebert W. The results of the titanium-coated RM acetabular component at 20 years: a term follow-up of an uncemented primary total hip replacement. J Bone Joint Surg (Br). 2008;90(1):1284–90..
Second generation monoblock uncemented cups were introduced in 2002 (Fig. 1b). Once again made from conventional PE, the difference was that the two pegs were no longer used. The longevity of the first two generations of monoblock cups was limited by wear and oxidation of the polyethylene [11] Erivan R, Eymond G, Villatte G, Mulliez A, Myriam G, Descamps S, et al. RM Pressfit® cup: good preliminary results at 5 to 8 years follow-up for 189 patients. Hip Int. 2016;26:386–91..
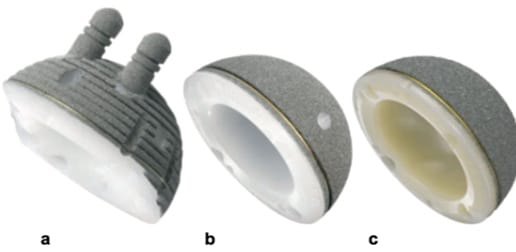
The third generation was released to market in 2009 (Fig. 1c), and these used highly cross-linked polyethylene with vitamin E added to improve mechanical and tribological properties [12] Beck M, Delfosse D, Lerf R, Becker R, French G. Oxidation prevention with vitamin E in a HXLPE isoelastic monoblock pressfit cup: preliminary results. In: Knahr K, editor. Total hip arthroplasty. Heidelberg: Springer; 2012. p. 21–31.. Early results from centres of expertise [13] Wyatt M, Weidner J, Pfluger D, Beck M. The RM Pressfit vitamys: 5-year Swiss experience of the first 100 cups. Hip Int. 2017;27:368–72. were promising and a number of other publications went on to confirm these hopes in the medium term [14], Scemama C, Anract P, Dumaine V, Babinet A, Courpied JP, Hamadouche M. Does vitamin E-blended polyethylene reduce wear in primary total hip arthroplasty: a blinded randomized clinical trial. Int Orthop. 2017;41: 1113–8.[15] Mahmood FF, Beck M, de Gast A, Rehbein P, French GJ, Becker R, Dominkus M, Helmy N, Hollmann L, Baines J. Survivorship and Patient-Reported Outcomes of an Uncemented Vitamin E-Infused Monoblock Acetabular Cup: A Multicenter Prospective Cohort Study. J Arthroplasty. 2021 May;36(5):1700-1706. . Afghanyar et al. [16] Afghanyar Y, Joser S, Tecle J, Drees P, Dargel J, Rehbein P, Kutzner KP. The concept of a cementless isoelastic monoblock cup made of highly cross-linked polyethylene infused with vitamin E: radiological analyses of migration and wear using EBRA and clinical outcomes at mid-term follow-up. BMC Musculoskelet Disord. 2021 Jan 23;22(1):107. reported on the data from a group of 81 vitamin E-stabilised XLPE monoblock uncemented cups with 6.6 years of follow-up (4.3–8.5 years) looking at survival and radiological wear using the EBRA method (for 42 cups). In this time frame, there was no recorded case of revision due to loss of fixation, no osteolysis and an annual rate of wear measured at 0.06mm (0.0–0.17). These results for the third generation monoblock uncemented cups were also evaluated against those of two other comparators:
- Firstly, Rochcongar et al. [17] Rochcongar G, Buia G, Bourroux E, Dunet J, Chapus V, Hulet C. Creep and Wear in vitamin E-infused highly cross-linked polyethylene cups for Total hip Arthroplasty: a prospective randomized controlled trial. J Bone Joint Surg Am . 2018;100(2):107–14. and Massier et al. [18] Massier JRA, Van Erp JHJ, Snijders TE, Gast A. A vitamin E blended highly cross-linked polyethylene acetabular cup results in less wear: 6-year results of a randomized controlled trial in 199 patients. Acta Orthop. 2020 Dec;91(6):705-710. compared them to those for the second generation monoblock uncemented PE cup: the rates of wear reported after 3 and 6 years respectively were significantly lower for the additive vitamin E group.
- Then, in 2023, Afghanyar et al. [19] Afghanyar Y, Möller JH, Wunderlich F, Dargel J, Rehbein P, Gercek E, Drees P, Kutzner KP. An isoelastic monoblock cup versus a modular metal-back cup: a matched-pair analysis of clinical and radiological results using Einzel-Bild-Röntgen-Analyse software. Arch Orthop Trauma Surg. 2023 Sep 23. compared them to those of a modular titanium uncemented cup used with a first generation remelted XLPE liner. After an average of 6.1 years of follow-up, no radiological difference was noted at the acetabulum, no revisions had been performed, and the wear rates were equivalent (0.06mm/year for the monoblock group and 0.07mm/year for the modular group, p=0.243). The equivalent wear rate after this time frame confirmed the results from previous generations, compiled in a review by Halma et al. [20] Halma JJ, Vogely HC, Dhert WJ, Van Gaalen SM, de Gast A. Do monoblock cups improve survivorship, decrease wear, or reduce osteolysis in uncemented total hip arthroplasty? Clin Orthop. 2013 Nov;471(11):3572-80. .
In conclusion, while short- and medium-term results for these uncemented monoblock cups are promising, and data from Australian and Swedish registries confirm that use of these implants is growing, the majority of these publications still come from centres of expertise, and follow-up periods remain insufficient to detect potential failures due to the material.
XLPE and dual mobility components
The use of dual mobility cups has been growing over the past few years in the prevention or management of instability in primary and revision THR [21] Darrith B, Courtney PM, Della Valle CJ. Outcomes of dual mobility components in total hip arthroplasty: a systematic review of the literature. . The concept of PE is demanding for the mobile cup design because it needs to combine the “standard” resistance to wear with enough elasticity for the initial movement of the head travelling into the retention area, without causing permanent damage. These concerns have led to specific ex vivo testing [22] Malatray M, Roux JP, Gunst S, Pibarot V, Wegrzyn J. Highly crosslinked polyethylene: a safe alternative to conventional polyethylene for dual mobility cup mobile component. A biomechanical validation. Int Orthop 2017;41:507–12., which showed that the snap-fit of the femoral head did not produce more cracks in the retention area with remelted or annealed XLPE than conventional PE. The second important conclusion was that the remelted XLPE group showed significantly lower femoral head extraction force when cracks were present than the CPE or annealed XLPE groups. The clinical significance of this finding has not yet been determined.
Epinette et al. [23] Epinette JA, Harwin SF, Rowan FE, Tracol P, Mont MA, Chughtai M, Westrich GH. Early experience with dual mobility acetabular systems featuring highly cross-linked polyethylene liners for primary hip arthroplasty in patients under fifty five years of age: an international multi-centre preliminary study. Int Orthop 2017;41:543–50. assessed the medium-term performance of a dual mobility cup using XLPE that had been sequentially irradiated and annealed. This prospective multicentre study included 321 young patients (mean age 48 years). The authors did not report any true or instraprosthetic dislocation. Two cups underwent revision due to impingement between the neck and cup. The all-cause survival rates were 97.5 % after five years. No radiological data concerning wear were reported.
Based on data on 33 explanted dual mobility cups with XLPE (sequentially irradiated and annealed) that were revised in the short-term due to non-mechanical failures, D’Apuzzo et al. [24] D’Apuzzo MR, Koch CN, Esposito CI, Elpers ME, Wright TM, Westrich GH. Assessment of Damage on a Dual Mobility Acetabular System. J Arthroplasty 2016;31:1828–35. confirmed that although the movements were produced at the two articulations (large and small), those from the small articulation seemed to dominate. In addition, the retentive mechanism remained intact in these short follow-up periods, irrespective of cup size. DM liner retentivity was tested on the explanted components through extraction of the ball from the DM cup (equivalent of an intraprosthetic dislocation). The results showed no relationship between the duration of implantation (mean of 6 months, range 0.06–26) and the extraction force required.
In conclusion, while the long-term data for the latest generations of XLPE liner are not yet available, theories based on ex vivo testing suggest that second generation XLPE, whether sequentially annealed or infused with vitamin E, is likely to constitute a more appropriate material for dual mobility components in view of the preservation of its mechanical properties.
XLPE with antioxidant additives: medium-term results
A PE is characterised through a balance between its different properties, especially resistance to wear, resistance to oxidation and mechanical properties. Irradiation cross-linking as used in first generation XLPE means that wear can be reduced, but the process creates free radicals, which trigger a risk of secondary oxidation. For this reason, irradiation is followed by a thermal treatment. There are various types of thermal treatment, none of which will completely eradicate the residual free radicals, and additionally they are thought to lead to reduced resistance to fatigue, which is already compromised by cross-linking. One alternative to thermal treatment that can still stabilise the free radicals produced by cross-linking is to use an antioxidant, such as vitamin E (alpha-tocopherol) [25] Bracco P, Oral E. Vitamin E-stabilized UHMWPE for total joint implants: a review. Clin Orthop Relat Res 2011;469:2286–93.. The physiological role of vitamin E is to react with the free radicals of the cell membrane and protect polyunsaturated fatty acids from oxidation degradation. The same mechanism is thought to apply to oxidation reactions within the PE, which contains aliphatic saturated very long chains. In practice, the manufacturing process of PE may involve variations in terms of the type of PE resin, the type of antioxidant and its concentration, the ways in which antioxidants are incorporated into the PE (“blended” before consolidation of the PE or “infused” afterwards), and the cross-linking and sterilisation of the XLPE.
Preclinical investigations have shown, apart from the “expected” strong performance (perfectly biocompatible, prevents oxidation and resists wear while preserving satisfactory mechanical properties), that the biological properties of XLPE with antioxidant additives give rise to a number of questions (such as whether it has an anti-inflammatory or antibacterial role) [26] Lambert B, Neut D, van der Veen HC, Bulstra SK. Effects of vitamin E incorporation in polyethylene on oxidative degradation, wear rates, immune response, and infections in total joint arthroplasty: a review of the current literature. Int Orthop 2019;43:1549–57.. Information about how this material behaves in vivo has been obtained from studies on explanted components. Rowell et al. looked at 15 explanted vitamin E-stabilised PE components, with a limited follow-up period ranging from 3 days to 3 years, and showed that the number of free radicals fell over time and that oxidative phenomena appeared to be inhibited as a consequence [27] Rowell SL, Muratoglu OK. Investigation of surgically retrieved, vitamin E-stabilized, crosslinked UHMWPE implants after short-term in vivo service. J Biomed Mater Res B Appl Biomater 2016;104:1132–40..
Vitamin E-stabilised XLPE components were introduced clinically in 2008. Spece et al. [28] Spece H, Yarbrough RV, Kurtz SM. In Vivo Performance of Vitamin E Stabilized Polyethylene Implants for Total Hip Arthroplasty: A Review. brought together the results from 41 studies (35 cohorts, four case reports, and two analyses of explanted components), with follow-up periods ranging from 2 to 11 years (median of 5 years). Taking all the studies that reported penetration of the femoral head on radiography, the wear rates ranged from 0.01 to 0.14mm/year for vitamin E-stabilised XLPE groups and 0.01 to 0.21mm/year for control groups. Although extremely thorough, this review could not determine any discernible difference between the two methods of incorporating vitamin E into PE. Another recent meta-analysis by Wyatt et al. [29] Wyatt MC, Roberton A, Foxall-Smi M, Beswick AD, Kunutsor SK, Whitehouse MR. Does vitamin E highly-crosslinked polyethylene convey an advantage in primary total hip replacement? A systematic review and meta-analysis. Hip Int. 2020 Sep;30(5):598-608. included five studies with medium-term follow-up, and this showed that penetration of the femoral head was significantly lower in vitamin E-stabilised XLPE groups. Although these results may seem promising there was no statistically significant difference in terms of the rate of revision.
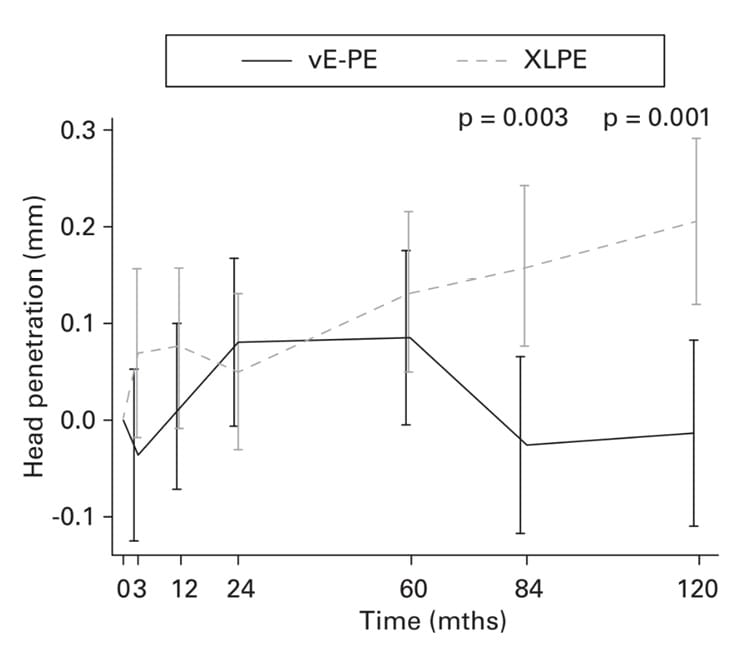
To our knowledge, the study into this material with the longest follow-up period published to date was by El-Sahoury et al. [30] El-Sahoury JAN, Kjærgaard K, Ovesen O, Hofbauer C, Overgaard S, Ding M. Vitamin E-diffused liners show less head penetration than cross-linked polyethylene liners in total hip arthroplasty: a ten-year multi-arm randomized trial. Bone Joint J. 2023 Oct 1;105-B(10):1052-1059. . Out of 116 randomised patients (vitamin E-infused XLPE versus mechanically annealed 5Mrad XLPE), 82 were reviewed (mean ages from 61 to 65 years) after a minimum of 10 years of follow-up: total mean penetration (RSA) was significantly lower for the vitamin E group (p=0.001), with no difference in terms of cup migration (Fig. 2). No cases of loosening or implant failure were reported. Moreover, this study did not note head size (32 versus 36mm) to have any significant impact on wear. Similar results, showing a significant difference in radiological wear with a mean of 7 years of follow-up but no repercussion on revision rates, were also reported by Collins et al. [31] Collins AK, Sauder N, Nepple CM, Blackburn AZ, Prasad AK, Feder OI, Melnic CM; Senior Authors Writing Committee; Bedair HS. Minimum 7-Year Follow-Up of Vitamin E-Diffused and Highly Cross-Linked Polyethylene Liners in Total Hip Arthroplasty: Findings From a Prospective, International, Multicenter Study of 977 Patients. J Arthroplasty. 2023 Nov;38(11):2373-2378. . In an original study, Mutsuoka et al. [32] Matsuoka T, Takahashi Y, Ishida T, Tateiwa T, Shishido T, Yamamoto K. In vivo creep and wear performance of vitamin-E-diffused highly crosslinked polyethylene in total hip arthroplasty. Arch Orthop Trauma Surg. 2023 Dec;143(12):7195-7203. carried out a comparative analysis looking at results from two groups of identical types of PE with additive vitamin E that differed in terms of the thickness of the PE (liner in an uncemented cup with a 28mm chrome-cobalt ball): 6.8mm versus 8.9mm. After 7 years of follow-up, the authors were unable to demonstrate any significant difference in wear with consistent use between the two groups. These results confirm the findings of Fransen et al. [33] Fransen BL, Howard LC, MacDonell T, Bengoa FJ, Sheridan GA, Garbuz DS, Neufeld ME. Highly Crosslinked Polyethylene Liner Thickness Does Not Influence Survival in Primary Total Hip Arthroplasty After Mean Follow-Up of 13 Years: A Study of 2,565 Hips With a Single Design Liner. J Arthroplasty. 2023 Jul;38(7 Suppl 2):S340-S345. , indicating after a mean of 13 years of follow-up that the thickness of a first generation XLPE component (9 Mrads, irradiated then remelted) had no influence on survivorship. These data could be used to justify fitting large diameter femoral heads.
Taken together, the results for XLPE with additive vitamin E are encouraging given that these studies document the behaviour of these materials in the long term (over 15 years), showing in particular a reduction in revisions due to aseptic loosening.
Chemical processes for XLPE cross-linking
At present, almost all XLPE is produced by ionising irradiation, mainly using gamma rays, to break the chains and release the free radicals needed for the cross-linking process. However, in view of a worldwide shortage in the supply of cobalt-60, a worrying fall in the availability of gamma radiation is likely to be imminent. For the growing industry of medical device sterilisation, regulatory pressure already tends to favour the alternative method of ethylene oxide (EtO) sterilisation. Technologies that do not rely on gamma radiation will also need to be developed and validated for cross-linking, as this will be crucial for XLPE to remain available. One promising method is to use peroxide as a chemical cross-linking agent.
Peroxide cross-linking has been proven to be effective in terms of increasing resistance to wear [34] Gul RM, Fung K, Doshi BN, Oral E, Muratoglu OK. Surface cross-linked UHMWPE using peroxides. J Orthop Res. 2017;35(11):2551-2556., but it does entail reduced oxidative stability, which has been controlled effectively by adding an antioxidant [35] Oral E, Doshi BN, Gul RM, Neils AL, Kayandan S, Muratoglu OK. Peroxide cross-linked UHMWPE blended with vitamin E. J Biomed Mater Res B Appl Biomater. 2017 Aug;105(6):1379-1389. . The biocompatibility of the product has also been tested in an animal model [36] Bichara DA, O'Brien CC, Doshi BN, Nielsen GP, Oral E, Muratoglu OK. Residual byproducts of peroxide crosslinked vitamin E-blended ultrahigh molecular weight polyethylene. J Arthroplasty. 2018;33(8):2666-2670..
More recently, Muratoglu et al. [37] Muratoglu OK, Asik MD, Nepple CM, Wannomae KK, Micheli BR, Connolly RL, Oral E. Di-cumyl peroxide cross-linked UHMWPE/vitamin-E blend for total joint arthroplasty implants. J Orthop Res. 2023 Aug 18. doi: 10.1002/jor.25679. Epub ahead of print. PMID: 37593816. studied a new vitamin E–UHMWPE blend with a more stable peroxide that is easy to use. The biological, tribological and mechanical results were positive, paving the way for FDA approval in January 2023, allowing this material to be used in tibial liners in TKR. Another team [38] Shah NA, Lan RT, Dai R, Jiang K, Shen HY, Hong R, Xu JZ, Li L, Li ZM. Improved oxidation stability and crosslink density of chemically crosslinked ultrahigh molecular weight polyethylene using the antioxidant synergy for artificial joints. J Biomed Mater Res B Appl Biomater. 2023 Jan;111(1):26-37. recently showed that a combination of antioxidants (vitamin E + D-Sorbitol) could offer an ideal solution in preparing XLPE through chemical cross-linking.
What can we learn from the rare XLPE implant failures?
The rare cases of XLPE implant failures have until recently always been reported to be related to implantation errors (excessive tilt, poor anteversion), traumas, dislocation or prosthesis impingements, or even use of a PE liner that is too thin (<5mm). The two cases of fractured XLPE described below occurred in the absence of these errors.
Wahl et al. [39] Wahl P, Mossu-Haas C, Dommann-Scherrer C, Wei K, Eschbach L, Gehr P, Benninger E. Early failure of a highly cross-linked polyethylene inlay after total hip arthroplasty probably due to insufficient irradiation. Proc Inst Mech Eng H. 2022 Dec;236(12):1711-1719. reported on a case of early pain (6 months after THR in a 49-year-old female, with implants oriented and integrated correctly) that led to multiple investigations, which did not find any infection or psoas impingement, but ultimately a strong macrophage activation against polyethylene particles in the micron size range. There was no three-body abrasive wear noted, and in view of the very short time scale of this wear that was thought to be from the liner, material-specific failure was diagnosed, followed by component explantation and thorough testing. Tests on the explanted component identified insufficient irradiation of the polyethylene as being the most probable cause of the failure, based on the transvinylene index. However, the gel content, crystallinity, fusion temperature and oxidation index all remained within expected ranges. This case highlights the importance of histological assessment, even in aseptic revision, as well as a detailed analysis of the explanted component in unexplained failures.
In contrast to two earlier cases reporting failures of vitamin E-infused XLPE liners [40], Bates MD, Mauerhan DR. Early fracture of a vitamin-E-infused, highly cross-linked polyethylene liner after total hip arthroplasty. JBJS Case Connect 2015;5:e65.[41] Brazier BG, Mesko JW. Superior rim fracture of a vitamin E-infused highly cross-linked polyethylene (HXLPE) liner leading to total hip arthroplasty revision. Arthroplast Today 2018;4:287–90., attributed either to insufficient thickness, a positioning error or a trauma, Kim et al. [42] Kim KB, Lee SM, Moon NH, Do MU, Shin WC. Early unexpected failure of a vitamin E-infused highly cross-linked polyethylene liner: A case report. Medicine (Baltimore). 2021 Oct 15;100(41):e27454. doi: 10.1097/MD.0000000000027454. PMID: 34731119; PMCID: PMC8519234. presented an unusual case of a fracture of the superior rim of a vitamin E-infused XLPE component, close to an area of excessive wear at the locking mechanism. This is an interesting case because this early failure of a PE liner 18 months after it was fitted occurred without repeated stresses or trauma (only sitting down and getting up), and the liner was relatively thick (5.8 mm at the periphery) and fitted correctly (46° of tilt and 18° of anteversion). It is therefore worth underlining the importance of perfectly mastering the snap-fit of the liner in a metal cup, and subsequently looking out for any unusual symptoms that patients may describe (such as an “explosion” in one case), and finally to remember that if there is any doubt, radiography or CT imaging should be performed to check for eccentration of the femoral head (Fig. 3).
Can the benefits of XLPE in hip implants be applied to the knee?
The biomechanics of the knee differ from those of the hip, meaning that the clinical successes with XLPE in THR have not been possible to reproduce directly in TKR. This can be explained by the fact that in TKR the polyethylene liner is submitted to a very different kind of force from in THR. The rolling, sliding and rotation mechanisms of the knee carry a higher risk of the components separating or fracture due to fatigue, and this is increased with PE ageing and oxidation, in contrast to the more consistent force in the hip [43] Brown TS, Van Citters DW, Berry DJ, Abdel MP. The use of highly crosslinked polyethylene in total knee arthroplasty. Bone Joint J. 2017;99-B(8):996–1002..
This means that usage of XLPE in TKR remains open to question. A number of studies of explanted components over the course of the past 15 years have reported equivalent results in terms of the patterns of wear of conventional PE and XLPE [44] Liu T, Esposito C, Elpers M, Wright T. Surface damage is not reduced with highly crosslinked polyethylene tibial inserts at short-term. Clin Orthop. 2016;474 (1):107–116.. It is likely that no difference was found in this study because the early revision was not caused by PE wear. Yu et al. [45] Yu BF, Yang GJ, Wang WL, Zhang L, Lin XP. Cross-linked versus conventional polyethylene for total knee arthroplasty: a meta-analysis. J Orthop Surg Res. 2016 Mar 30;11:39. looked at six studies in a meta-analysis that compared CPE and XLPE over a period of 2 to 6 years, evaluating the revision rate for all causes, whether loosening, osteolysis or mechanical failure. They found no statistically significant difference. These results were confirmed by other authors [46], Meneghini RM, Ireland PH, Bhowmik-Stoker M. Multicenter study of highly cross-linked versus conventional polyethylene in total knee arthroplasty. J Arthroplasty. 2015;10(4):34–1016.[47] Wilhelm SK, Henrichsen JL, Siljander M, Moore D, Karadsheh M. Polyethylene in total knee arthroplasty: where are we now? J Orthop Surg. 2018;26(3), 2309499018808356., and in a more recent meta-analysis of 10 studies covering 963,467 patients [48] Gkiatas I, Karasavvidis T, Sharma AK, Xiang W, Malahias MA, Chalmers BP, Sculco PK. Highly cross-linked polyethylene in primary total knee arthroplasty is associated with a lower rate of revision for aseptic loosening: a meta-analysis of 962,467 cases. Arch Orthop Trauma Surg. 2022 Jun;142(6):1177-1184. . Kendall et al. have also added to the questions over the value of XLPE in TKR [49] Kendall J, Pelt CE, Imlay B, Yep P, Mullen K, Kagan R. No Reduction in Revision Risk Associated With Highly Cross-linked Polyethylene With or Without Antioxidants Over Conventional Polyetheylene in TKA: An Analysis From the American Joint Replacement Registry. Clin Orthop. 2022 Oct 1;480(10):1929-1936. , although a response from McKellop [50] McKellop HA. CORR Insights®: No Reduction in Revision Risk Associated With Highly Cross-linked Polyethylene With or Without Antioxidants Over Conventional Polyethylene in TKA: An Analysis From the American Joint Replacement Registry. Clin Orthop. 2022 Oct 1;480(10):1937-1939. drew attention to various methodological weaknesses in the analysis of Kendall et al., with one point being that in view of the multiple factors that can influence loss of fixation in a TKR, the PE liner alone cannot be incriminated in a retrospective analysis that is not particularly rigorous and mixes different types of XLPE.
Only a few studies have looked at in vivo use of vitamin E-stabilised XLPE liners in TKR. Spece et al. [51] Spece H, Yarbrough RV, Kurtz SM. A Review of Early In Vivo Performance of Antioxidant Stabilized Polyethylene for Total Knee Arthroplasty. compiled the clinical data from 13 studies (five of which were cohort studies with follow-up periods ranging from 2 to 7 years). In these short time frames, no mechanical failures were reported, with equivalent survival rates and identical radiological parameters (wear, periprosthetic radiolucency, osteolysis). Analyses of explanted components confirmed the protection from oxidation.
Conclusion
There is steady and encouraging progress in the development of polyethylene types amid a climate of pressing economic considerations (reducing healthcare costs) and geopolitical setbacks (lack of available cobalt-60). Although the in vitro or short-term results are broadly positive compared to those of previous generations, we should remain cautious on certain points:
- Each PE is a product of a specific manufacturing process (irradiation and sterilisation methods, antioxidants or other additives) and should be evaluated individually. Simplifying analyses by grouping into “generations” may be a dangerous short cut;
- At present, it is reasonable to warn against using conventional PE for primary THR; however, in primary TKR there is no reliable data on the basis of which we can routinely recommend using XLPE, irrespective of the manufacturing process;
- Caution must prevail when using PE liners, even second generation components, concerning in particular the design (elastic retention area, insufficient thickness at the periphery or the snap-fit site) and the positioning (tilt, anteversion) of implants;
- As performance standards for THR have reached an extremely high level, any change must be backed up by solid evidence (including long-term results from research teams that are not the designers) before generalisations can be made.
References
1. García-Rey E, Cruz-Pardos A, Saldaña L. New polyethylenes in total hip arthroplasty: a 20- to 22-year follow-up study. Bone Joint J. 2022 Sep;104-B(9):1032-1038.
2. Orita K, Goto K, Kuroda Y, Kawai T, Okuzu Y, Matsuda S. Wear resistance of first-generation highly cross-linked annealed polyethylene in cementless total hip arthroplasty is maintained 20 years after surgery. Bone Joint J. 2022 Feb;104-B(2):200-205.
3. de Steiger R, Lorimer M, Graves SE. Cross-Linked Polyethylene for Total Hip Arthroplasty Markedly Reduces Revision Surgery at 16 Years. J Bone Joint Surg Am 2018;100:1281–8.
4. Yoon BH, Park JW, Lee YK, Koo KH, Chang CB. Long-Term Wear-Related Complications of Cross-Linked Versus Conventional Polyethylene After Total Hip Arthroplasty: A Meta-Analysis. J Arthroplasty. 2022 Nov;37(11):2308-2315.e2.
5. Deans CF, Buckner BC, Garvin KL. Wear, Osteolysis, and Aseptic Loosening Following Total Hip Arthroplasty in Young Patients with Highly Cross-Linked Polyethylene: A Review of Studies with a Follow-Up of over 15 Years. J Clin Med. 2023 Oct 19;12(20):6615.
6. Pakvis D, Biemond L, van Hellemondt G, Spruit M. A cementless elastic monoblock socket in young patients: a ten to 18-year clinical and radiological follow-up. Int Orthop. 2011;35:1445–51.
7. Pitto RP, Bhargava A, Pandit S, Munro JT. Retroacetabular stress-shielding in THA. Clin Orthop. 2008 Feb;466(2):353-8.
8. Meneghini RM, Ford KS, McCollough CH, Hanssen AD, Lewallen DG. Bone remodeling around porous metal cementless acetabular components. J Arthroplasty. 2010 Aug;25(5):741-7.
9. Brodt S, Jacob B, Nowack D, Zippelius T, Strube P, Matziolis G. An Isoelastic Monoblock Cup Retains More Acetabular and Femoral Bone Than a Modular Press-Fit Cup: A Prospective Randomized Controlled Trial. J Bone Joint Surg Am . 2021 Jun 2;103(11):992-999.
10. Ihle M, Mai S, Pfluger D, Siebert W. The results of the titanium-coated RM acetabular component at 20 years: a term follow-up of an uncemented primary total hip replacement. J Bone Joint Surg (Br). 2008;90(1):1284–90.
11. Erivan R, Eymond G, Villatte G, Mulliez A, Myriam G, Descamps S, et al. RM Pressfit® cup: good preliminary results at 5 to 8 years follow-up for 189 patients. Hip Int. 2016;26:386–91.
12. Beck M, Delfosse D, Lerf R, Becker R, French G. Oxidation prevention with vitamin E in a HXLPE isoelastic monoblock pressfit cup: preliminary results. In: Knahr K, editor. Total hip arthroplasty. Heidelberg: Springer; 2012. p. 21–31.
13. Wyatt M, Weidner J, Pfluger D, Beck M. The RM Pressfit vitamys: 5-year Swiss experience of the first 100 cups. Hip Int. 2017;27:368–72.
14. Scemama C, Anract P, Dumaine V, Babinet A, Courpied JP, Hamadouche M. Does vitamin E-blended polyethylene reduce wear in primary total hip arthroplasty: a blinded randomized clinical trial. Int Orthop. 2017;41: 1113–8.
15. Mahmood FF, Beck M, de Gast A, Rehbein P, French GJ, Becker R, Dominkus M, Helmy N, Hollmann L, Baines J. Survivorship and Patient-Reported Outcomes of an Uncemented Vitamin E-Infused Monoblock Acetabular Cup: A Multicenter Prospective Cohort Study. J Arthroplasty. 2021 May;36(5):1700-1706.
16. Afghanyar Y, Joser S, Tecle J, Drees P, Dargel J, Rehbein P, Kutzner KP. The concept of a cementless isoelastic monoblock cup made of highly cross-linked polyethylene infused with vitamin E: radiological analyses of migration and wear using EBRA and clinical outcomes at mid-term follow-up. BMC Musculoskelet Disord. 2021 Jan 23;22(1):107.
17. Rochcongar G, Buia G, Bourroux E, Dunet J, Chapus V, Hulet C. Creep and Wear in vitamin E-infused highly cross-linked polyethylene cups for Total hip Arthroplasty: a prospective randomized controlled trial. J Bone Joint Surg Am . 2018;100(2):107–14.
18. Massier JRA, Van Erp JHJ, Snijders TE, Gast A. A vitamin E blended highly cross-linked polyethylene acetabular cup results in less wear: 6-year results of a randomized controlled trial in 199 patients. Acta Orthop. 2020 Dec;91(6):705-710.
19. Afghanyar Y, Möller JH, Wunderlich F, Dargel J, Rehbein P, Gercek E, Drees P, Kutzner KP. An isoelastic monoblock cup versus a modular metal-back cup: a matched-pair analysis of clinical and radiological results using Einzel-Bild-Röntgen-Analyse software. Arch Orthop Trauma Surg. 2023 Sep 23. doi: 10.1007/s00402-023-05058-8. Epub ahead of print. PMID: 37740060.
20. Halma JJ, Vogely HC, Dhert WJ, Van Gaalen SM, de Gast A. Do monoblock cups improve survivorship, decrease wear, or reduce osteolysis in uncemented total hip arthroplasty? Clin Orthop. 2013 Nov;471(11):3572-80.
21. Darrith B, Courtney PM, Della Valle CJ. Outcomes of dual mobility components in total hip arthroplasty: a systematic review of the literature. Bone Joint J. 2018 Jan;100-B(1):11-19.
22. Malatray M, Roux JP, Gunst S, Pibarot V, Wegrzyn J. Highly crosslinked polyethylene: a safe alternative to conventional polyethylene for dual mobility cup mobile component. A biomechanical validation. Int Orthop 2017;41:507–12.
23. Epinette JA, Harwin SF, Rowan FE, Tracol P, Mont MA, Chughtai M, Westrich GH. Early experience with dual mobility acetabular systems featuring highly cross-linked polyethylene liners for primary hip arthroplasty in patients under fifty five years of age: an international multi-centre preliminary study. Int Orthop 2017;41:543–50.
24. D’Apuzzo MR, Koch CN, Esposito CI, Elpers ME, Wright TM, Westrich GH. Assessment of Damage on a Dual Mobility Acetabular System. J Arthroplasty 2016;31:1828–35.
25. Bracco P, Oral E. Vitamin E-stabilized UHMWPE for total joint implants: a review. Clin Orthop Relat Res 2011;469:2286–93.
26. Lambert B, Neut D, van der Veen HC, Bulstra SK. Effects of vitamin E incorporation in polyethylene on oxidative degradation, wear rates, immune response, and infections in total joint arthroplasty: a review of the current literature. Int Orthop 2019;43:1549–57.
27. Rowell SL, Muratoglu OK. Investigation of surgically retrieved, vitamin E-stabilized, crosslinked UHMWPE implants after short-term in vivo service. J Biomed Mater Res B Appl Biomater 2016;104:1132–40.
28. Spece H, Yarbrough RV, Kurtz SM. In Vivo Performance of Vitamin E Stabilized Polyethylene Implants for Total Hip Arthroplasty: A Review.
J Arthroplasty. 2023 May;38(5):970-979. doi: 10.1016/j.arth.2022.11.010. Epub 2022 Dec 5. PMID: 36481286.
29. Wyatt MC, Roberton A, Foxall-Smi M, Beswick AD, Kunutsor SK, Whitehouse MR. Does vitamin E highly-crosslinked polyethylene convey an advantage in primary total hip replacement? A systematic review and meta-analysis. Hip Int. 2020 Sep;30(5):598-608.
30. El-Sahoury JAN, Kjærgaard K, Ovesen O, Hofbauer C, Overgaard S, Ding M. Vitamin E-diffused liners show less head penetration than cross-linked polyethylene liners in total hip arthroplasty: a ten-year multi-arm randomized trial. Bone Joint J. 2023 Oct 1;105-B(10):1052-1059.
31. Collins AK, Sauder N, Nepple CM, Blackburn AZ, Prasad AK, Feder OI, Melnic CM; Senior Authors Writing Committee; Bedair HS. Minimum 7-Year Follow-Up of Vitamin E-Diffused and Highly Cross-Linked Polyethylene Liners in Total Hip Arthroplasty: Findings From a Prospective, International, Multicenter Study of 977 Patients. J Arthroplasty. 2023 Nov;38(11):2373-2378.
32. Matsuoka T, Takahashi Y, Ishida T, Tateiwa T, Shishido T, Yamamoto K. In vivo creep and wear performance of vitamin-E-diffused highly crosslinked polyethylene in total hip arthroplasty. Arch Orthop Trauma Surg. 2023 Dec;143(12):7195-7203.
33. Fransen BL, Howard LC, MacDonell T, Bengoa FJ, Sheridan GA, Garbuz DS, Neufeld ME. Highly Crosslinked Polyethylene Liner Thickness Does Not Influence Survival in Primary Total Hip Arthroplasty After Mean Follow-Up of 13 Years: A Study of 2,565 Hips With a Single Design Liner. J Arthroplasty. 2023 Jul;38(7 Suppl 2):S340-S345.
34. Gul RM, Fung K, Doshi BN, Oral E, Muratoglu OK. Surface cross-linked UHMWPE using peroxides. J Orthop Res. 2017;35(11):2551-2556.
35. Oral E, Doshi BN, Gul RM, Neils AL, Kayandan S, Muratoglu OK. Peroxide cross-linked UHMWPE blended with vitamin E. J Biomed Mater Res B Appl Biomater. 2017 Aug;105(6):1379-1389.
36. Bichara DA, O'Brien CC, Doshi BN, Nielsen GP, Oral E, Muratoglu OK. Residual byproducts of peroxide crosslinked vitamin E-blended ultrahigh molecular weight polyethylene. J Arthroplasty. 2018;33(8):2666-2670.
37. Muratoglu OK, Asik MD, Nepple CM, Wannomae KK, Micheli BR, Connolly RL, Oral E. Di-cumyl peroxide cross-linked UHMWPE/vitamin-E blend for total joint arthroplasty implants. J Orthop Res. 2023 Aug 18. doi: 10.1002/jor.25679. Epub ahead of print. PMID: 37593816.
38. Shah NA, Lan RT, Dai R, Jiang K, Shen HY, Hong R, Xu JZ, Li L, Li ZM. Improved oxidation stability and crosslink density of chemically crosslinked ultrahigh molecular weight polyethylene using the antioxidant synergy for artificial joints. J Biomed Mater Res B Appl Biomater. 2023 Jan;111(1):26-37.
39. Wahl P, Mossu-Haas C, Dommann-Scherrer C, Wei K, Eschbach L, Gehr P, Benninger E. Early failure of a highly cross-linked polyethylene inlay after total hip arthroplasty probably due to insufficient irradiation. Proc Inst Mech Eng H. 2022 Dec;236(12):1711-1719.
40. Bates MD, Mauerhan DR. Early fracture of a vitamin-E-infused, highly cross-linked polyethylene liner after total hip arthroplasty. JBJS Case Connect 2015;5:e65.
41. Brazier BG, Mesko JW. Superior rim fracture of a vitamin E-infused highly cross-linked polyethylene (HXLPE) liner leading to total hip arthroplasty revision. Arthroplast Today 2018;4:287–90.
42. Kim KB, Lee SM, Moon NH, Do MU, Shin WC. Early unexpected failure of a vitamin E-infused highly cross-linked polyethylene liner: A case report. Medicine (Baltimore). 2021 Oct 15;100(41):e27454. doi: 10.1097/MD.0000000000027454. PMID: 34731119; PMCID: PMC8519234.
43. Brown TS, Van Citters DW, Berry DJ, Abdel MP. The use of highly crosslinked polyethylene in total knee arthroplasty. Bone Joint J. 2017;99-B(8):996–1002.
44. Liu T, Esposito C, Elpers M, Wright T. Surface damage is not reduced with highly crosslinked polyethylene tibial inserts at short-term. Clin Orthop. 2016;474 (1):107–116.
45. Yu BF, Yang GJ, Wang WL, Zhang L, Lin XP. Cross-linked versus conventional polyethylene for total knee arthroplasty: a meta-analysis. J Orthop Surg Res. 2016 Mar 30;11:39.
46. Meneghini RM, Ireland PH, Bhowmik-Stoker M. Multicenter study of highly cross-linked versus conventional polyethylene in total knee arthroplasty. J Arthroplasty. 2015;10(4):34–1016.
47. Wilhelm SK, Henrichsen JL, Siljander M, Moore D, Karadsheh M. Polyethylene in total knee arthroplasty: where are we now? J Orthop Surg. 2018;26(3), 2309499018808356.
48. Gkiatas I, Karasavvidis T, Sharma AK, Xiang W, Malahias MA, Chalmers BP, Sculco PK. Highly cross-linked polyethylene in primary total knee arthroplasty is associated with a lower rate of revision for aseptic loosening: a meta-analysis of 962,467 cases. Arch Orthop Trauma Surg. 2022 Jun;142(6):1177-1184.
49. Kendall J, Pelt CE, Imlay B, Yep P, Mullen K, Kagan R. No Reduction in Revision Risk Associated With Highly Cross-linked Polyethylene With or Without Antioxidants Over Conventional Polyetheylene in TKA: An Analysis From the American Joint Replacement Registry. Clin Orthop. 2022 Oct 1;480(10):1929-1936.
50. McKellop HA. CORR Insights®: No Reduction in Revision Risk Associated With Highly Cross-linked Polyethylene With or Without Antioxidants Over Conventional Polyethylene in TKA: An Analysis From the American Joint Replacement Registry. Clin Orthop. 2022 Oct 1;480(10):1937-1939.
51. Spece H, Yarbrough RV, Kurtz SM. A Review of Early In Vivo Performance of Antioxidant Stabilized Polyethylene for Total Knee Arthroplasty. J Arthroplasty. 2023 Sep;38(9):1885-1891.

