bioball® adapter system: a supportive modular tool for primary and revision hip arthroplasty
Background: Restoring native hip biomechanics, particularly femoral offset and leg length, can be challenging in both primary and revision total hip arthroplasty (THA). Instability due to insufficient soft-tissue tension and isolated acetabular revisions with a well-integrated femoral stem are common scenarios where standard implants may be inadequate.
Objective of the Article: This article presents the technical characteristics, indications, surgical technique, and clinical outcomes of the BioBall® modular taper adapter system as a solution for complex hip reconstruction.
Key Points / Core Message: The BioBall® system is a versatile adapter-head system that allows for intraoperative adjustment of neck length, offset, and femoral version. Its primary indication is in revision THA, particularly for isolated acetabular component exchange, where it facilitates biomechanical correction while retaining a well-fixed femoral stem. The system is available for various taper sizes and can be combined with different stem materials and head types. A critical step is the intraoperative assessment of the existing stem taper; the system is contraindicated for tapers with significant damage, such as palpable defects or material loss, as this increases failure risk. Surgeons must also consider the increased lever arm created by extra-long adapters, which can elevate stress on the implant, especially when used with modular revision stems.
Conclusion / Implications for Practice: The BioBall® modular adapter system is an effective tool that provides intraoperative flexibility to address complex biomechanical challenges in THA. It can prevent the need for a more extensive femoral stem revision, which is particularly beneficial in high-risk patients. While clinical studies report favorable long-term outcomes and low complication rates, meticulous surgical technique, including rigorous taper evaluation, is paramount to ensure successful and durable reconstruction.
Introduction
Total hip arthroplasty (THA) is one of the most successful surgical procedures worldwide. Recent studies show a 5% increase in the longevity of total hip prostheses compared to the results from ten years ago [1] Clar, C., et al., The worldwide survival rate of total hip arthroplasties is improving: a systematic comparative analysis using worldwide hip arthroplasty registers. EFORT Open Rev, 2024. 9(8): p. 745-750.. However, the annual global rate of primary total hip replacements continues to rise, indicating an overall future increase in revision procedures as well.
In primary operations as well as revision THA surgeons may face challenging anatomical and procedural situations. Appropriate reconstruction of leg length and offset is not always possible with standard implants. Especially in patients with a very long femoral neck, implantation of a standard-sized stem can lead to reduction of the femoral offset, which may result in painful abductor dysfunction and even instability. Not only in primary, but also in revision surgery, one of the most common early postoperative complications is instability with resulting dislocation of the hip joint [2], Melvin, J.S., et al., Early failures in total hip arthroplasty - a changing paradigm. J Arthroplasty, 2014. 29(6): p. 1285-8.[3], Karachalios, T., G. Komnos, and A. Koutalos, Total hip arthroplasty: Survival and modes of failure. EFORT Open Rev, 2018. 3(5): p. 232-239.[4] Hermansen, L.L., et al., "True" Cumulative Incidence of and Risk Factors for Hip Dislocation within 2 Years After Primary Total Hip Arthroplasty Due to Osteoarthritis: A Nationwide Population-Based Study from the Danish Hip Arthroplasty Register. J Bone Joint Surg Am, 2021. 103(4): p. 295-302.. The causes can be multifactorial, ranging from the previously described pathology to further patient-related aspects such as pre-existing neurological disorders, prior surgeries, or poor compliance and even surgeon-induced implant malposition. With increasing patient age in primary procedures and multiple previous surgeries in revision cases a frequent reason is insufficient soft-tissue tension, where extra-long heads may be necessary to achieve a stable articulation.
Another challenging situation is isolated revision of acetabular cups either due to loosening or wear of bearing partners, where the femoral component is well integrated. After component exchange on the acetabular side, a new femoral head needs to be placed on the taper of the remaining stem. Studies have demonstrated that ceramic heads must not be re-used on previously used tapers, due to a significantly increased risk of failure of the new head [5] Pautasso, A., et al., Usefulness of modular neck adapter in partial hip revision. Annals of Joint, 2023. 8: p. 35-35.. Likewise, reattaching metal heads onto damaged tapers may lead to fretting corrosion and subsequent trunnionosis. Therefore, it may be necessary to apply special ceramic heads with integrated titanium sleeves or even special adapters. Especially in excessive wear situations with necessary resection of granulomatous soft tissue masses, it may be challenging to achieve stability.
For all these situations in primary as well as revision THA modular taper adapters may provide a promising solution.
Characteristics of the bioball® system
The BioBall® system (Merete®, Germany) is a modular adapter-head system that enables intraoperative adjustments in neck length, offset shift, and to a certain degree, also torsional alignment of the femur (ante-/retrotorsion). It also provides the surgeon with the possibility to exchange the head in isolated acetabular revision surgery, where the femoral stem can be retained.
System Overview and Materials:
The BioBall® system offers 11 adapter configurations as well as combinations with different head types. The 12/14 taper is available from size S to 5XL, providing eight potential length adjustments from -3 mm up to +21 mm (Figure 1). This broad spectrum allows for a wide range of clinical scenarios. The adapter system is also available for 14/16 taper configurations (seven length adjustments from 0mm up to +21mm).

Another feature of the BioBall® system is the availability of offset adapters, which enable not only length correction but also medial or lateral displacement of the center of rotation. In addition, the 7.5° offset allows rotation in any plane and thereby limited torsional adjustment. Depending on the adapter size (M to 5XL), seven extensions of up to +21 mm are possible for 12/14 tapers (Figure 2a). For 14/16 tapers, four adjustments are still available (Figure 2b), ranging from size 2XL (+10,5mm) to 5XL (+21,0mm). Offset adjustments can be made in increments ranging from 1.1 mm to 3.0 mm (12/14 taper) and 1,4 mm to 2,5mm (14/16 taper), respectively. Optimizing the center of rotation helps regulate soft tissue tension, thereby reducing complications such as dislocations and impaired muscular force transmission.
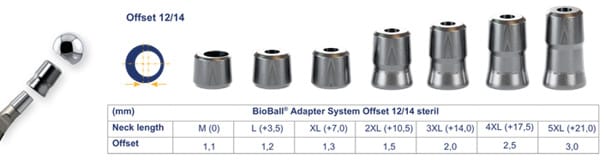
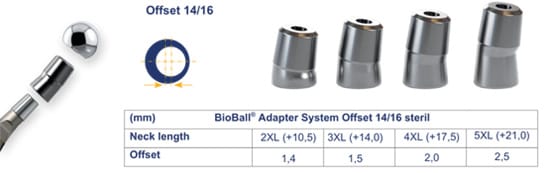
Apart from 12/14 and 14/16 tapers in the standard tray, other taper kinds (8/10, 10/12, 11/12, 11/13, and V40) are also available on request. These features result in a very high versatility of the system.
All adapters are made from a high-strength titanium alloy (TiAl6V4 ELI, DIN EN ISO 5832-3). They can be combined with stems made of
- Titanium (TiAl6V4 as well as TiAl6Nb7)
- CoCr alloys (DIN ISO 5832-4/-12)
- Stainless Steel (DIN ISO 5832-9)
The adapters must be used only with Bioball® femoral heads. Various modular head components (Figure 3) as well as sizes are available:
- BioBall® Metal Heads (Vivium®)
- BioBall® DELTA® Ceramic Heads (BIOLOX® delta)
- Standard head sizes 28, 32, 36, 40 mm (Metal and Ceramic)
- Additional sizes for Ceramic Heads (40, 44, and 48 mm) as well as Metal Heads (sizes 33, 35, and 38 mm) available on request

Regarding the bearing partners, metal heads can only be combined with PE-/XLPE inlays, while ceramic heads can be used with PE/XLPE- and/or BIOLOX® delta inlays.
Indications and contraindications
Application of the BioBall® system can be indicated in primary as well as revision hip surgery.
Indications
Primary THA: necessary intraoperative adaptation of offset, neck length, lateralisation and anteversion/retroversion (i.e. in cases where appropriate anatomic reconstruction and stability cannot be achieved with standard long and extra-long heads) - applies only to 12/14 adapters.
Revision THA:
- bearing couple revisions
- intraoperative correction of offset, neck length, lateralization and anteversion/retroversion of stems which are retained and not exchanged
- isolated exchange of head and acetabular cup and/or inlay in well osteointegrated stems
- combination with revision stem, if stability and/or appropriate leg length cannot be achieved with standard head
Contraindications
- Acute or chronic infections in the hip joint or the immediate vicinity
- Patients with joint disorders, that may be successfully treated with another salvage procedure
- Severe comorbidities with a risk to the function or success of the implant (i.e. neuromuscular or vascular disorders of the involved leg)
- Severely damaged in-situ stem tapers (visible changes in shape, or palpable defects, such as localised wear, abrasion/material loss, or scratches/ridges)
- Implants with taper size, which cannot be clearly identified
- Allergies to any of the materials used
Manufacturer’s indications and contraindications are listed in instructions for use (IFU) [6] Merete GmbH, BioBall™ Adapter System Surgical Instructions 09.2023.. To ensure the compatibility between the Merete BioBall® Adapter System and the stems from other manufactures, it is mandatory to use the Merete BioBall® AdapterSelector® for verification. If the appropriate Adapter is selected, surgeons should consider explaining the rationale behind their use to their patients and obtain their informed consent prior to the surgeries.
Selection of taper adapter
Preoperatively, radiographic templating as well as control of implant ID (revisions) is advised to select appropriate and compatible components. In revision cases without stem exchange, the surgeon must check intra-operatively for taper size and potential damage. After removal of the previous head, it is necessary to verify compatibility between the implant's taper and the 12/14 adapte. For this purpose, the BioBall® AdapterSelector® is placed over the cleaned taper. A correct fit is confirmed when no gap is visible between the AdapterSelector® and the taper and the taper tip lies between the two reference lines. A smooth rotational movement is then used to test proper seating (Figure 4).
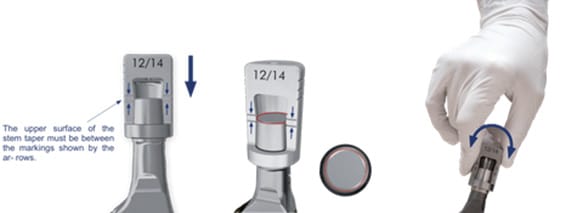
It is further crucial to ensure that the taper has no significant damage. After thorough rinsing and cleaning of the in situ stem taper, potential damage is assessed. In case of visible changes in shape or palpable defects due to significant wear or abrasion, which is associated with material loss, the application of an adapter is contraindicated. The intra-operative “finger test” can be helpful: the surgeon runs a finger along the taper surface; if significant irregularities are detected, the taper is deemed unsuitable for use with an adapter. The manufacturer of the BioBall® system also advises not to use the system, if taper color has changed in an area of more than 10% of taper surface, which indicates superficial metal corrosion.
If taper integrity is assured, different trial components can be tested to select the appropriate adapter length (Figure 5). Stability and neck length / offset are assessed (if necessary, under fluoroscopic control).
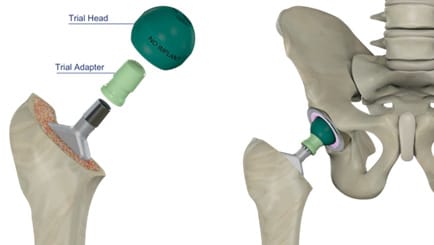
If the application of an offset adapter is planned, selection of appropriate positioning of the trial component as well as the final implant is possible via a marking system (Figure 6). The 12 o’clock position represents maximum lateralization or medialization. There is also an “Offset Position Assistant” available to simulate the optimal adapter alignment in terms of CCD angle and torsion.

After final selection of the appropriate adapter, assembly can be performed.
First, it is necessary to apply BioBall® Adapter to the stem taper with axial pressure in the selected angle (CCD or antetorsion).
Next, mount the head on the BioBall® Adapter with axial pressure. Check the correct seating of the head and adapter. Fix the head with a light hammer blow in axial direction by using the head impactor. To avoid damage, never strike the adapter or the head with a hammer directly. Use only ceramic heads and not metal heads in revision surgery after ceramic component breakage to prevent damage from ceramic debris.
Potential pitfalls to consider
Through its versatility, the BioBall® Adapter system provides excellent solutions for many clinical scenarios in primary as well as revision THA. Nevertheless, certain aspects must be considered to avoid complications.
- As always in modular arthroplasty implants, there is a certain risk of fretting and corrosion at the adapter-taper interface. Increased lever arms as well as friction moments can lead to material conflict. Careful cleaning of surfaces and stable assembly according to IFU are crucial to avoid complications.
- Adapters should not be used in implants with substantial damage of the taper surface. Although different scoring systems are available to classify taper damage [7], Hothi, H.S., et al., Damage Patterns at the Head-Stem Taper Junction Helps Understand the Mechanisms of Material Loss. J Arthroplasty, 2017. 32(1): p. 291-295.[8] Higgs, G.B., et al., Does Taper Size Have an Effect on Taper Damage in Retrieved Metal-on-Polyethylene Total Hip Devices? J Arthroplasty, 2016. 31(9 Suppl): p. 277-81. it is difficult to define a threshold for adapter applicability. At least in cases with visible changes in shape or palpable taper defects due to significant wear or abrasion, which are associated with material loss, the application of an adapter is contraindicated.
- Overall lengthening of the neck length on the femoral side by application of extra-long adapters increases the lever arm on the implant itself. This may result in critical load, especially if modular revision stems are used [9] Bormann, T., et al., Is taper corrosion in modular revision hip stem junctions associated with patient or implant specific factors? A retrieval analysis. Journal of the Mechanical Behavior of Biomedical Materials, 2024. 150.. Potential fractures of taper connections in modular revision implants are rare but well-known complications. Krueger et al. [10] Krueger, D.R., et al., Mechanical failure of 113 uncemented modular revision femoral components. Bone Joint J, 2020. 102-B(5): p. 573-579. analyzed 113 implant fractures occurring at the modular stem junction of a revision stem. In 79% of cases, the stem had been combined with a lateralized neck segment, an extra-long head, or both. They also reported that extension sleeves with double modular junctions had been used more frequently in cases with failed implants, although this did not reach significance. As large head offset is one critical factor in modular revision stem overload, most manufacturers recommend in their IFU to avoid extra-long heads and/or adapter systems which exceed certain length. In revision cases with substantial soft tissue defects, however, it is often difficult to achieve stability without application of longer adapters.
In primary THA as well as revision arthroplasty, surgeons must be aware of potential medicolegal issues. By definition, the combination of implants or parts of implants which are produced by different manufacturers, is considered a “mix & match” application. Therefore, the combination of a BioBall® Adapter system with stems from other manufacturers falls into this category, if there is no explicit approval in IFUs. The advantages of the Adapter system with regard to reduced operating time, less blood loss and preserved bone stock over the alternative of a stem exchange in order to avoid a mix & match situation cannot be overemphasized. Recently published EFORT-recommendations, which apply to such situations, have therefore explicitly confirmed the applicability of taper adapters and other re-sleeving devices in revision surgery [11] Tucker, K., et al., EFORT recommendations for off-label use, mix & match and mismatch in hip and knee arthroplasty. EFORT Open Rev, 2021. 6(11): p. 982-1005.. When these devices are used off-label, where possible, surgeons should explain the rationale behind their use and obtain informed consent. In addition, it is mandatory to respect the integrity of the taper of retained stem as well as its compatibility with the selected adapter (i.e. by using the BioBall® AdapterSelector® as mentioned above).
Clinical results
Novoa et al. [12] Novoa, C.D., et al., The Merete BioBall system in hip revision surgery: A systematic review. Orthopaedics & Traumatology: Surgery & Research, 2018. 104(8): p. 1171-1178. published a systematic review summarizing available investigations regarding the BioBall® Adapter system up to 2017. They concentrated on indications for its use, clinical results and causes of secondary revision surgery. Isolated acetabular revision was the main indication for implant use. When prior dislocation was the indication of adapter application, the rate of second revision seemed to be high. There was no report of neck adapter failure or corrosion phenomena. Considering a limited data base in general, however, the authors concluded that convincing data reports were lacking for a definitive recommendation regarding the use of the adapter system and advocated for further prospective controlled trials.
The results of some individual studies, which have been included in the review, shall briefly be summarized. Hohberg et al. [13] Hoberg, M., et al., Outcome of a modular head-neck adapter system in revision hip arthroplasty. Arch Orthop Trauma Surg, 2015. 135(10): p. 1469-74. evaluated postoperative satisfaction in a cohort of 95 patients who underwent acetabular revision combined with head exchange to a BioBall® system, with a mean follow-up of 52.5 months. Clinical outcomes were assessed, among other parameters, using the Harris Hip Score. The mean score was 80.9 points (range 33–100). Overall, 89.4% of patients reported satisfaction with the surgical outcome, whereas only 1.1% expressed subjective dissatisfaction. Caternicchia et al. [14] Caternicchia, F., et al., Revision Hip Arthroplasty Using a Modular Head–Neck Adapter System and a Ceramic Head: 5-Year Clinical and Radiographic Outcomes. Journal of Clinical Medicine, 2023. 12(14). found that over a mean follow-up of 59.8 ± 26 months, no mechanical complications occurred with the adapter in combination with a ceramic head, regardless of whether a 12/14, a 14/16, or an offset adapter was used on an intact taper. Likewise, in the only hip, which had to be revised for recurrent aseptic cup loosening, no macroscopic signs of mechanical failure, including fretting corrosion or implant breakage, were observed. In the study by Helwig et al. [15] Helwig, P., et al., Modular sleeves with ceramic heads in isolated acetabular cup revision in younger patients-laboratory and experimental analysis of suitability and clinical outcomes. Int Orthop, 2013. 37(1): p. 15-9., first- to second-degree taper damage did not result in ceramic head fracture during a mean follow-up of 26 months in young patients undergoing primary acetabular revision. Dickinson et al. [16] Dickinson, E.C., K. Sellenschloh, and M.M. Morlock, Impact of stem taper damage on the fracture strength of ceramic heads with adapter sleeves. Clin Biomech (Bristol), 2019. 63: p. 193-200. however demonstrated that using highly damaged tapers significantly increased adapter fracture rates, especially in combination with ceramic heads.
Since 2017, there are multiple investigations published confirming the applicability of the BioBall® Adapter system: In a study by Dabis et al. [17] Dabis, J., et al., Clinical outcomes and dislocation rates after hip reconstruction using the Bioball system. Hip Int, 2020. 30(5): p. 609-616. involving patients undergoing revision total hip arthroplasty, retention of a well-integrated femoral stem was achieved in 83% of cases through the use of the BioBall® Adapter. The adapters were employed for various reasons: in approximately half of the patients, a modified taper geometry for changing the ante- or retroversion was utilized, while in the remaining cases, the indication was intraoperative adjustment of offset or leg length. Pardo et al. [18] Pardo, F., et al., A Modular Head-Neck Adapter System and Ceramic Heads in Revision Hip Arthroplasty: A Registry Study on 354 Implants. J Arthroplasty, 2023. 38(8): p. 1578-1583. compared, in a cohort study, a modular adapter system in combination with a ceramic head (354 implantations) and a metal head (395 implantations) with regard to reasons for revision and head size. At the 5-year follow-up, the mean survival rate was 87.9%, at 7 years 86.9%, and at 10 years 85.7%.
In a retrospective series of 45 patients with an ASA score of II–IV, outcomes with the BioBall® Adapter were assessed over a 10-year follow-up [19] Garabadi, M., et al., Clinical outcome of Bioball universal adapter in revision hip arthroplasty. . Revision surgery was performed for aseptic loosening in 44.6% and for instability in 40.4% of cases. At final follow-up, no material failures were observed. Only one case of recurrent instability was noted, attributable to subsequent multisegmental lumbar fusion. Consistent results were reported by Kock et al. in 2020 [20] Kock, H.-J., et al., Long-term outcome after revision of hip arthroplasty with the BioBall® adapter system in multimorbid patients. Journal of Orthopaedic Translation, 2020. 22: p. 43-49., who investigated 19 multimorbid patients treated with a BioBall® Adapter between 2002 and 2004. In addition to implant survival, patient-reported quality of life was assessed. Over a follow-up period of up to 12 years, a revision rate of 14% was observed. The Barthel Index remained stable, with a mean score of 79 ± 26 in 2004 (median 90 [65-100]) and 89 ± 20 in 2011 (median 100 [95-100]). These findings suggest that preservation of the femoral stem is particularly advantageous in elderly and multimorbid patients, as it is associated with reduced operative time and blood loss.
Conclusion
The BioBall® Adapter System is a well-established and highly modular tool in modern endoprosthetics. The system’s strengths are intraoperative flexibility, material versatility, and high biomechanical adaptability. Particularly in complex revision procedures - and even in selected primary cases - it enables individualized treatment without the need for full stem revision. Multiple clinical examples confirm excellent long-term stability and functionality. The system has a very low rate of complications as reported in the current literature. In particular, no corrosion or fretting of the modular system has been reported. Nevertheless, the dataset is still limited, and further investigations are necessary.
Clinical Case Studies
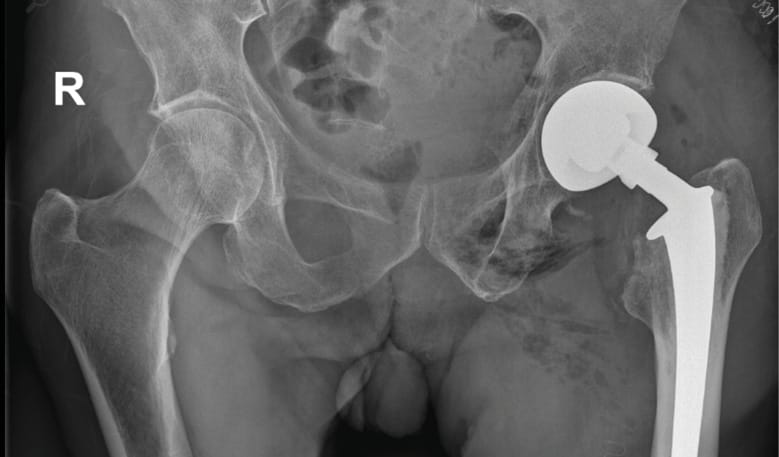
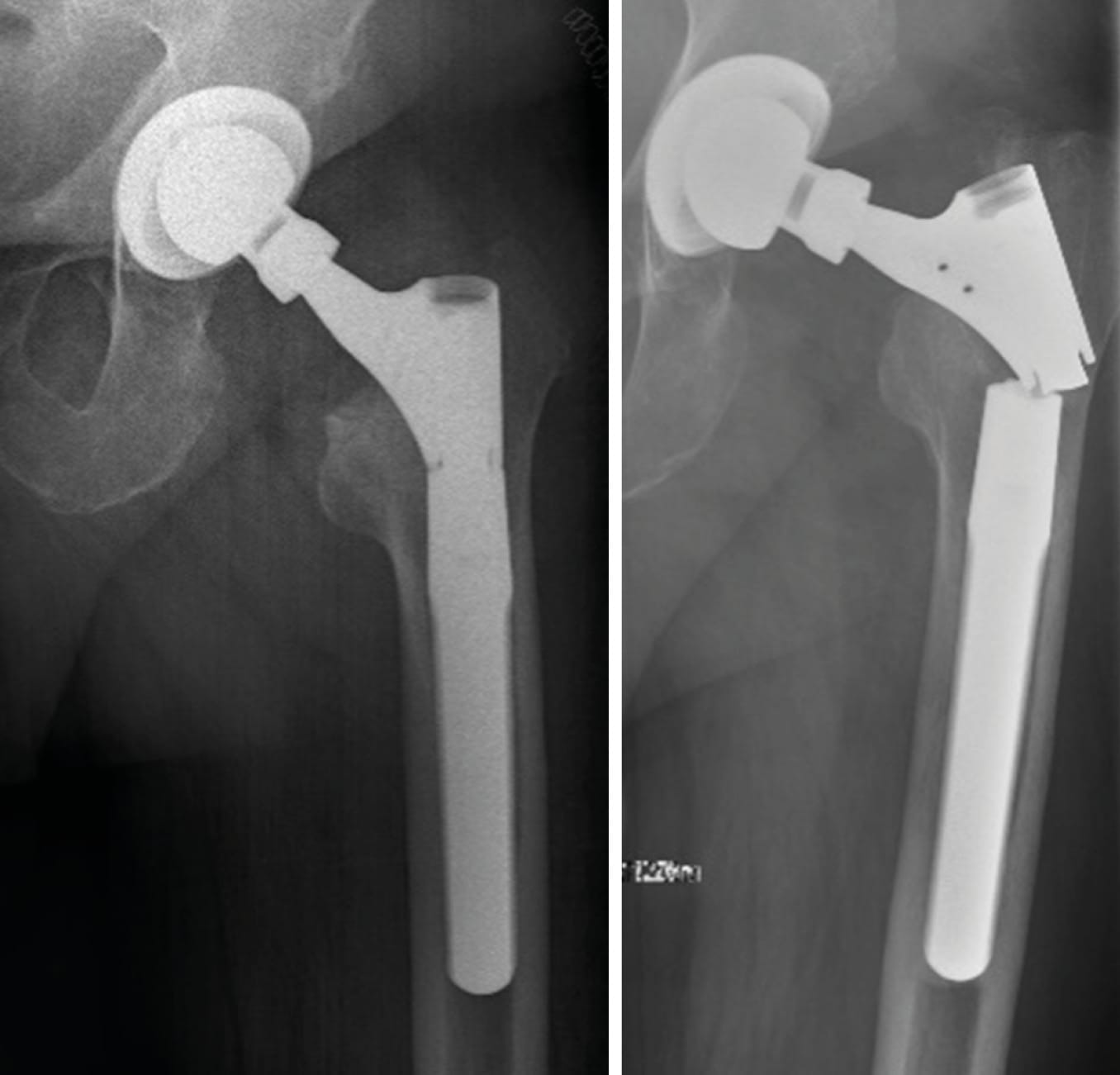
Case 1: Instability after primary cementless total hip arthroplasty (32-year-old female)
A female patient with recurrent dislocations following a cementless primary total hip arthroplasty (THA) was treated using head exchange with BioBall® 2XL standard adapter. The offset correction improved soft tissue tension and joint stability. No further dislocations were observed during a 10-year follow-up.

Case 2: Femoral neck fracture in elderly patient with poor soft tissue tension and large offset (91-year-old female)
Traumatic fragility fracture of the left femoral neck. Due to a large offset, the original anatomy could not be restored by hemiarthroplasty using a lateralized stem alone. After several intra-operative trials, soft tissue tension could adequately be balanced with the application of a BioBall® 4XL adapter. A stable articulation without any further instability could be accomplished.
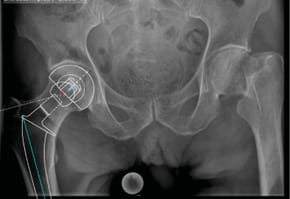
Case 3: Acetabular cup revision due to aseptic loosening (62-year-old female)
Progressive loosening and cranial migration of an uncemented threaded cup with a Paprosky type 3b (“up-and-in”) bone defect. After removal of the loose cup, reconstruction of the defect was performed with a cemented antiprotrusio cage in combination with impaction bone grafting along the medial wall. As the stem was found to be well osteointegrated, offset and neck length were restored with a Bioball® standard adapter 5XL and a ceramic head. At 10-year follow-up, radiographs showed no loosening.
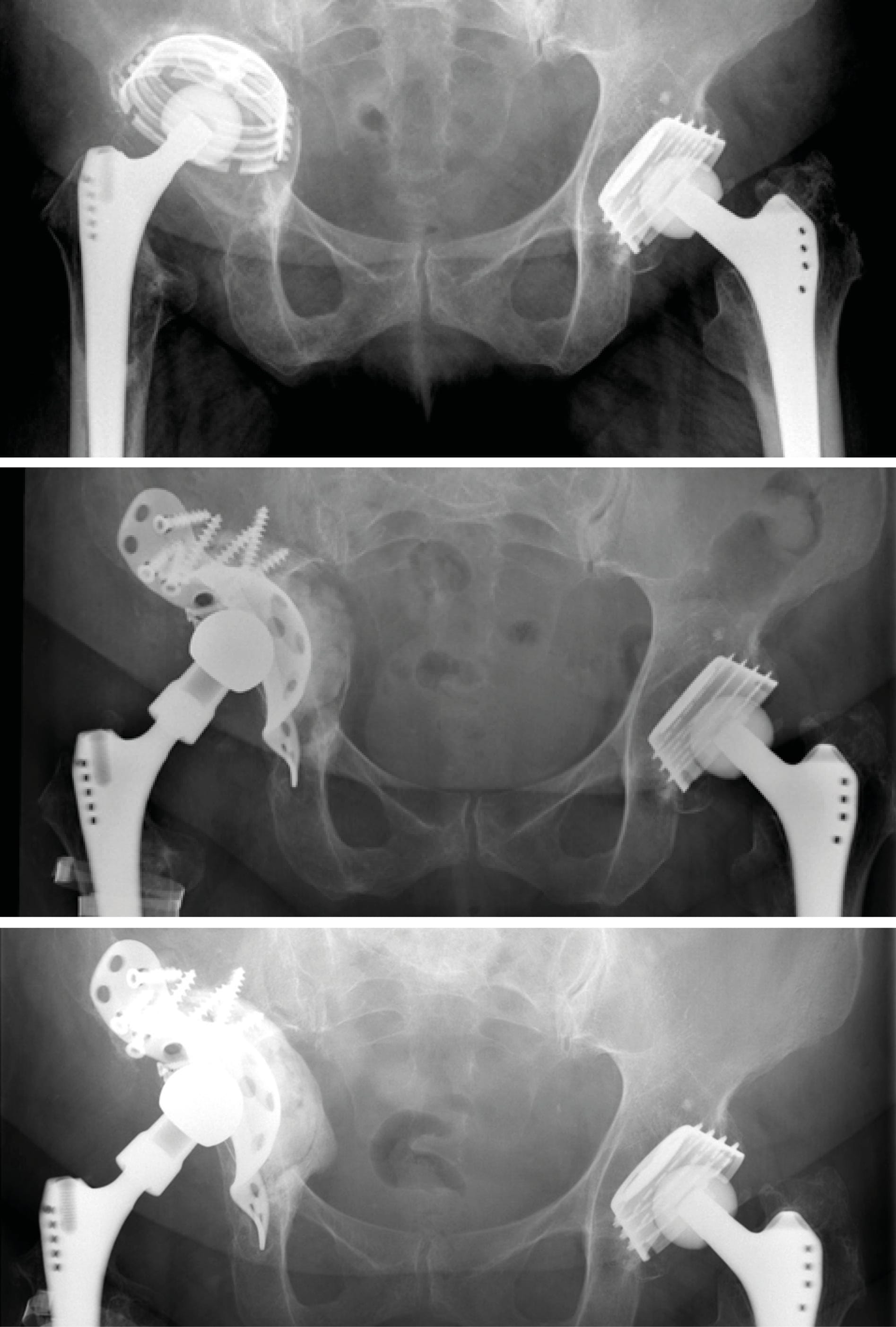
Case 4: Acetabular cup revision in pelvic discontinuity (86-year-old female)
Reconstruction of pelvic discontinuity and recurrent dislocations due to medial migration of an unstable cemented reinforcement ring with an off-label application of cage and augment in combination with a cemented dual mobility cup. Neck length and offset were restored with a Bioball® standard adapter 3XL and a ceramic head.
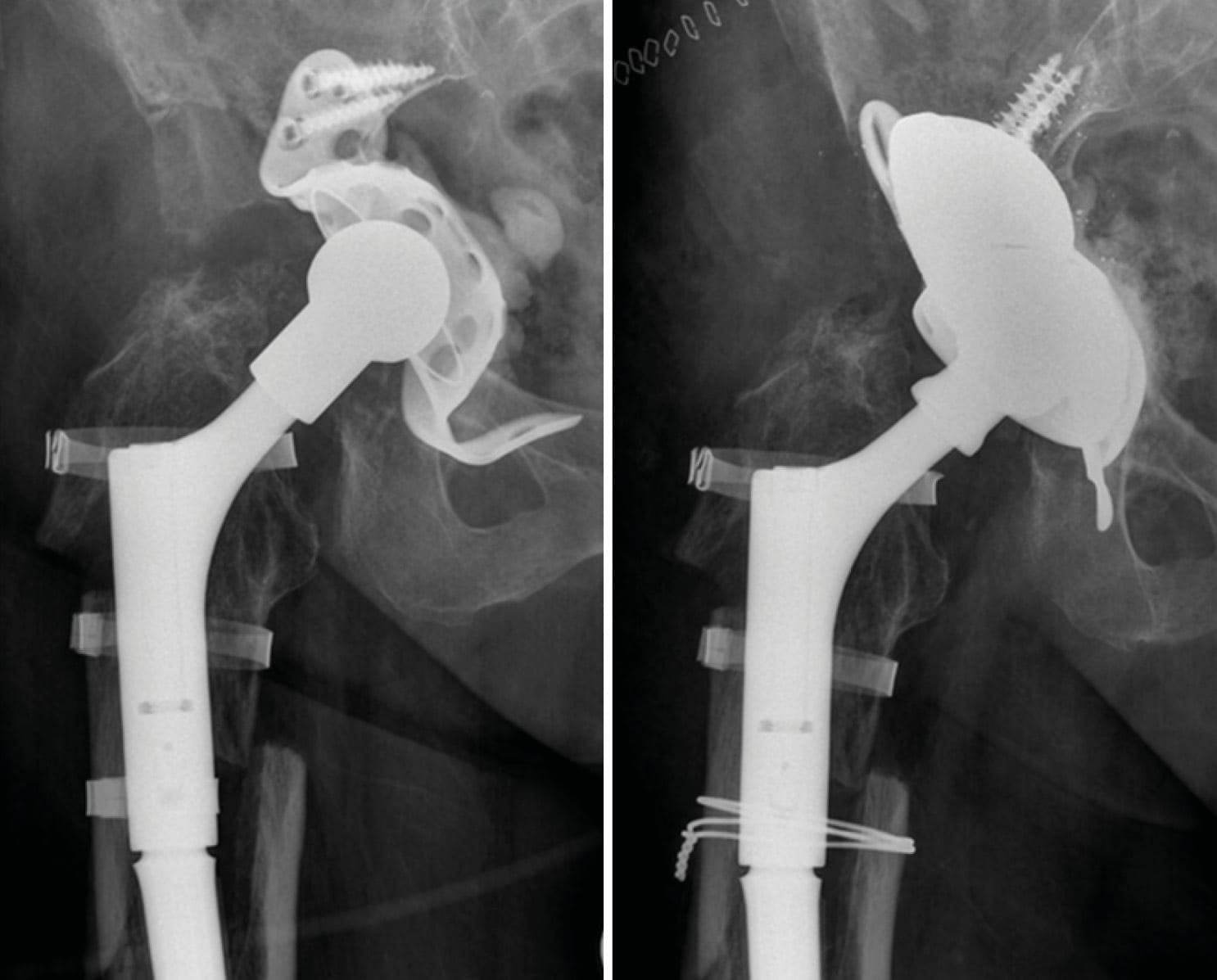
Case 5: Caution with application of extra-long adapters and modular revision stems (76-year-old male)
The patient had undergone femoral revision surgery due to aseptic stem loosening of the left hip 5 years ago. He subsequently developed failure of the taper junction of the modular revision stem. Most manufacturers advise against using heads larger than size L in combination with modular revision stems due to increased lever arms which may exceed material tolerance of taper connections.
References
1. Clar, C., et al., The worldwide survival rate of total hip arthroplasties is improving: a systematic comparative analysis using worldwide hip arthroplasty registers. EFORT Open Rev, 2024. 9(8): p. 745-750.
2. Melvin, J.S., et al., Early failures in total hip arthroplasty - a changing paradigm. J Arthroplasty, 2014. 29(6): p. 1285-8.
3. Karachalios, T., G. Komnos, and A. Koutalos, Total hip arthroplasty: Survival and modes of failure. EFORT Open Rev, 2018. 3(5): p. 232-239.
4. Hermansen, L.L., et al., "True" Cumulative Incidence of and Risk Factors for Hip Dislocation within 2 Years After Primary Total Hip Arthroplasty Due to Osteoarthritis: A Nationwide Population-Based Study from the Danish Hip Arthroplasty Register. J Bone Joint Surg Am, 2021. 103(4): p. 295-302.
5. Pautasso, A., et al., Usefulness of modular neck adapter in partial hip revision. Annals of Joint, 2023. 8: p. 35-35.
6. Merete GmbH, BioBall™ Adapter System Surgical Instructions 09.2023.
7. Hothi, H.S., et al., Damage Patterns at the Head-Stem Taper Junction Helps Understand the Mechanisms of Material Loss. J Arthroplasty, 2017. 32(1): p. 291-295.
8. Higgs, G.B., et al., Does Taper Size Have an Effect on Taper Damage in Retrieved Metal-on-Polyethylene Total Hip Devices? J Arthroplasty, 2016. 31(9 Suppl): p. 277-81.
9. Bormann, T., et al., Is taper corrosion in modular revision hip stem junctions associated with patient or implant specific factors? A retrieval analysis. Journal of the Mechanical Behavior of Biomedical Materials, 2024. 150.
10. Krueger, D.R., et al., Mechanical failure of 113 uncemented modular revision femoral components. Bone Joint J, 2020. 102-B(5): p. 573-579.
11. Tucker, K., et al., EFORT recommendations for off-label use, mix & match and mismatch in hip and knee arthroplasty. EFORT Open Rev, 2021. 6(11): p. 982-1005.
12. Novoa, C.D., et al., The Merete BioBall system in hip revision surgery: A systematic review. Orthopaedics & Traumatology: Surgery & Research, 2018. 104(8): p. 1171-1178.
13. Hoberg, M., et al., Outcome of a modular head-neck adapter system in revision hip arthroplasty. Arch Orthop Trauma Surg, 2015. 135(10): p. 1469-74.
14. Caternicchia, F., et al., Revision Hip Arthroplasty Using a Modular Head–Neck Adapter System and a Ceramic Head: 5-Year Clinical and Radiographic Outcomes. Journal of Clinical Medicine, 2023. 12(14).
15. Helwig, P., et al., Modular sleeves with ceramic heads in isolated acetabular cup revision in younger patients-laboratory and experimental analysis of suitability and clinical outcomes. Int Orthop, 2013. 37(1): p. 15-9.
16. Dickinson, E.C., K. Sellenschloh, and M.M. Morlock, Impact of stem taper damage on the fracture strength of ceramic heads with adapter sleeves. Clin Biomech (Bristol), 2019. 63: p. 193-200.
17. Dabis, J., et al., Clinical outcomes and dislocation rates after hip reconstruction using the Bioball system. Hip Int, 2020. 30(5): p. 609-616.
18. Pardo, F., et al., A Modular Head-Neck Adapter System and Ceramic Heads in Revision Hip Arthroplasty: A Registry Study on 354 Implants. J Arthroplasty, 2023. 38(8): p. 1578-1583.
19. Garabadi, M., et al., Clinical outcome of Bioball universal adapter in revision hip arthroplasty.
J Orthop, 2023. 38: p. 68-72.
20. Kock, H.-J., et al., Long-term outcome after revision of hip arthroplasty with the BioBall® adapter system in multimorbid patients. Journal of Orthopaedic Translation, 2020. 22: p. 43-49.

